Detta inlägg post publicerades ursprungligen på denna sida this site ;
Date:
Author: Paulomi (Polly) Burey, Professor in Food Science, University of Southern Queensland
Original article: https://theconversation.com/is-there-a-best-way-to-peel-a-boiled-egg-a-food-scientist-explains-235895
We’ve all been there – trying to peel a boiled egg, but mangling it beyond all recognition as the hard shell stubbornly sticks to the egg white. Worse, the egg ends up covered in chewy bits of adhesive membrane in the end.
The internet is littered with various “hacks” that claim to prevent this problem. But there are several reasons why eggs can be hard to peel. Luckily, that means there are also science-based strategies we can use to avoid the problem.
Egg ‘peelability’ factors
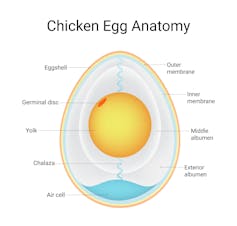
Eggs consist of a hard, porous shell, an inner and outer membrane, the egg white (albumen), and a membrane-encased yolk at the centre. There is also an air cell between the inner and outer membrane next to the shell.
Twinkle Picture/Shutterstock
A lot of research was done in the late 1960s and 1970s on factors that affect the peelability of eggs after they’ve been boiled.
One of these factors is the pH of the egg white. An early study from the 1960s indicated that the pH of the egg white needs to be in the range of 8.7–8.9, quite alkaline, in order for the egg to be easier to peel.
Storage temperature has a role to play, too. A study from 1963 showed that storing eggs at about 22 degrees Celsius (or 72 degrees Fahrenheit) gives a better peelability result than storage at lower temperatures of 13°C, or even fridge temperatures at 3–5°C.
Of course, there is a risk of spoilage if eggs are stored at higher ambient temperatures.
In the studies, an increase in storage time before boiling – using less fresh eggs – also increased the ease of peelability.

Caroline Attwood/Unsplash
Step one: avoid fresh eggs
The fact that fresh eggs are harder to peel is relatively well known. Based on the factors above, there are a couple of reasons for this.
For one, in a fresh egg the air cell is still quite small. As the egg ages, it (very) slowly loses moisture through the porous shell, increasing the size of the air cell while the rest of the egg contents shrink. A bigger air cell makes it easier to start the peeling action.
Additionally, egg whites, although they already start out relatively alkaline, increase in pH as the eggs age, also making them easier to peel.
Step two: water temperature
Some keen egg boiling pundits believe that starting off with boiling water and lowering it to a simmer before gently placing the eggs into it provides a better result. However, you want to do this with room temperature eggs to avoid them cracking due to a sudden temperature change.
The reasoning behind this approach is that exposure to higher temperatures from the start of cooking also makes it easier for the membrane to come away from the shell and egg white.
Furthermore, the quick hot start makes it easier for the egg white proteins to denature (change structure as they cook) and bond to each other, rather than to the membrane.
After boiling eggs for the desired amount of time (typically 3–5 minutes for runny yolks, 6–7 minutes for jammy yolks, and 12–15 minutes for hard boiled), you can quench them in ice water. This should help the egg white to slightly shrink away from the shell, improving peelability.

Max4e Photo/Shutterstock
Step three (optional): adding things to the water
Some other suggestions to improve peelability include adding salt to the boiling water, but this has mixed results. In one study, this approach did actually improve peelability, but this effect was lost after eggs had been stored for longer periods.
Acids and alkali have also been shown to aid eggshell peelability or removal. The patent that describes this used rather harsh substances with the goal to dissolve away the shell.
But based on this idea, you could try adding baking soda or vinegar to the water. With vinegar, the theory is that it attacks the calcium carbonate in the eggshell to then aid its removal. As for baking soda, because it’s alkaline, it could help detach the membrane from the shell.
Bonus: alternative cooking methods
There are other methods for hard-cooking eggs, such as pressure steaming, air-frying and even microwaving.
In steaming eggs, some proponents theorise that water vapour permeates the eggshell, loosening the membrane from the egg white, and thereby making the egg much easier to peel.
While studies have recently been done on the air-frying of other foods, there is still scope to further understand how this style of cooking might affect eggshells and peelability.
Lastly, once you have successfully separated the eggshells, don’t just throw them in the bin. There are lots of different uses for them, including compost, slug and snail deterrent in your garden, using them as little biodegradable pots for seedlings, or even something as advanced as scaffolds for cancer research.
![]()
Paulomi (Polly) Burey receives funding from the Australian Government Department of Education which has funded the eggshell research mentioned at the end of this article.

